Near-Infrared Absorbing Materials

閉じる
Search
Near-infrared absorbing materials combine high visible light transparency with strong selective absorption against near-infrared light. For example, by applying it to window materials, the energy of near-infrared rays contained in sunlight is efficiently cut while maintaining sufficient brightness, resulting in an effect that greatly suppresses the temperature rise in the room.
LaB6 (lanthanum hexaboride) and CWO™ (cesium-dope tungsten oxide) *, which are materials that absorb near-infrared rays developed by Sumitomo Metal Mining Metal Mining Co., Ltd., selectively absorb more near-infrared rays contained in sunlight than conventional materials such as ITO (indium-dope tin oxide) and ATO (antimony-dope tin oxide).
*CWO™ is a registered trademark of Sumitomo Metal Mining.
Sunlight consists of ultraviolet rays (UVC: ~290 nm, UVB:290 to 320 nm, UVA:320 to 380 nm), visible rays (380 to 780 nm), near infrared rays (780 to 2500 nm), and mid-infrared rays (2500 to 4000 nm). Its energy ratio is 7% for ultraviolet rays, 47% for visible rays, and 46% for near-and mid-infrared rays. Near-infrared rays (hereafter abbreviated as NIR) have a higher radiation intensity at shorter wavelengths, and they penetrate the skin and have a higher heat-generating effect, so they are also called “heat rays.”
Heat absorbing glass or heat reflecting glass is generally used to shield window glass from solar radiation. Heat-absorbing glass is made by NIR-absorption of iron (Fe) components, etc. kneaded into glass, and can be manufactured inexpensively. However, visible light transparency cannot be sufficiently ensured because it has a color tone peculiar to the material. Heat-reflective glass, on the other hand, attempts to reflect solar radiation energy by physically forming metals and metal oxides on the glass surface. However, the reflected wavelengths extend to visible light, which causes glare in appearance and radio interference. Dispersion of transparent conductors such as high-performance sunlight-shielding ITOs and ATOs with high visible light transparency and no radio wave disruption into nano-fine chemicals yields a transparency profile as shown in Fig. 1, and near-IR selective absorption membranes with radio wave transparency.
However, referring to the spectrum of sunlight, there is a large energy intensity around 800-1200 nm, and the fine particle dispersion film of ITO and ATO cannot remove this part sufficiently. LaB6 and CWO™ can cover light-absorption in the above-mentioned wavelength range, enabling more efficient removal of heat rays.
Fig.1 Transmittance profiles of typical nanoparticle dispersions
The shading effect of sunlight is expressed quantitatively in terms of the solar radiation heat acquisition rate (the fraction of net sunlight energy flowing through the glass) or the solar radiation shielding factor normalized by a 3 mm thick clear glass. Practically, it is preferable to use as bright a film as possible to reduce the solar radiation shielding coefficient as much as possible, and the result of plotting this relationship with respect to various materials is shown in Figure 2.
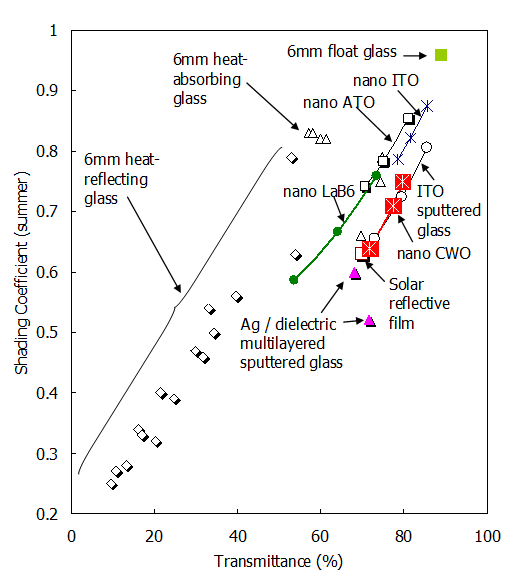
The lower right in Figure 2 indicates higher characteristics. This indicates that ATO, LaB6, and ITO fine particle dispersion films have better properties than heat-absorbing glass and heat-reflective glass. The fine particle dispersion film of CWO™ is even higher than these and has almost the same characteristics as those of ITO single-layer sputtered glass and M Co. multilayer heat-reflective film. Even if the absorption of heat rays is a coating film with a main function, it is an important point in industrial production to have a low-cost simple process with solar radiation shielding properties equivalent to those of a dry coating film with a strong reflection component.
In addition, LaB6 and CWO™ require very small amounts of materials to be used because of their large absorption coefficient per unit weight. Figure 3 compares the amount of fine particles (per unit projected area of the transparency) required to obtain the same solar radiation shielding factor. For LaB6 and CWO™, you can see that much less than ITO or ATO has the equivalent sun shield. For LaB6, the amount required to form a film with a visible light transmission of about 70% is only a 0.02 g/m2 and can be as helpful as 1/100 over ITO. Effectiveness with such a lean dispersion density can be a cost-effective advantage.
LaB6 is a black material used as an electronic source for TEMs. All rare earth elements hexaboride absorb near-infrared light, but we discovered and patented in 1997 that LaB6, a hexaboride of lanthanum, has the best heat-shielding ability.6-9) LaB6, unlike ATOs and ITOs, has a slightly green color tone, but it is characterized by high transparency and very low utilization for the sunlight heat acquisition rate (Fig. 3).
This is derived from the very high morphological absorptivity of LaB6. The large NIR-absorption principles of LaB6 originate from localized surface plasmon resonance exhibited by conductive nanoparticles, and their absorption wavelengths depend on the shapes of the particles.5)
Fig.3 Amount of usage of typical solar control nanoparticles
Fig.4 Absorption profiles of LaB6 nanoparticles of different diameters
Figure 4 shows the measured absorption profiles of different LaB6 dispersions with different mean particle sizes. When the average particle size is as large as 1.3μm, NIR-absorption around 1000 nm hardly occurs, but when the average particle size is as small as 326 nm, some absorption appears, and as further atomization advances, it can be seen that the absorption increases. In other words, this absorption is a phenomenon that occurs only when the particle size becomes nano-sized, and when the particle size decreases, it becomes stronger in a narrower wavelength range. However, it can be said that it actually has the largest absorptions when the particle size is around 90nm due to the surface-indexing of LaB6. LaB6 nanoparticles are also weatherproof against light and heat. However, they may slightly fade (decrease in absorbing power) after prolonged exposure to extreme temperatures and humidity. As a precaution against this problem, LaB6 particles that have been coated with a silico film and have improved damp resistance have been put into practical use.3)
CWO™ is a fine conductive particle obtained by adding cesium to tungsten trioxide (WO3) and is a new heat-ray absorbing material developed by us in 2004. 2,13-14)
WO3 is an insulator with monoclinic structures, but it is known that it becomes a conductor by oxygen-reduction or doping, which is a 10-11). WO3-x caused by oxygen deficiency due to reduction results in free electrons from apparently deficient oxygen, resulting in a conductor. As oxygen deficiency increases, WO3-x becomes a compound series called Magneli phase in which the edges of WO6 octahedra are shared at various places, and some of them exhibit high-electron-conductivity and remarkable NIR-absorbing properties.
Another way to make WO3 conductive is by adding a third element. The monolithic WO3 has regular gaps corresponding to the M-site of the perovskite structured MWO3, and a third element, such as Na, can be placed in the center of these gaps at various percentages. As the ionic radii of the additive elements increase, the crystallinity changes to cubic (Na, etc.), tetragonal (Ba, etc.), and hexagonal (K, Tl, Rb, Cs, etc.). Generally, the monovalent third element M, which is added to WO3, dissociates from M → M + e-in the crystals, and M+ enters the voids created by WO6 octahedra, while e-is placed around W6+ to form W5+. This electron hopping conduction improves the conductivity of WO3 and the polaron absorption of electrons causes NIR-absorbing properties. Alkaline elements also have a hexagonal tungsten bronze structure (hereinafter abbreviated as HTB) when K, Tl, Rb, or Cs with a large ionic radius are added. The crystal structure of HTB is shown in Figure 5. This is a structure in which WO6 octahedra are shared by edges, but a structure in which there are many tunneling-like voids that appear as hexagons or triangles on the bottom cut surface. When all regular hexagonal tunnels are filled with a third element, such as Cs, the atomic ratio of M to W is 0.33. When the crystallographic structure is HTB, the visible-light transmittance increases as shown in Fig. 6, and the 2,12) exhibits excellent heat-absorbing properties.
Sumitomo Metal Mining examined the production process of HTB fine particles with added cations, and it was possible to establish an industrially advantageous production method through optimization of raw material selection, manufacturing method, composition ratio, etc. We also found that the Cs-added system is the most industrially compatible combination among the HTBs studied in terms of characteristics, weather resistance, and cost. This combination is positioned as a new heat ray absorbing material of fine particle dispersion type exceeding the characteristics of ITO known until now.
Fig.5 Hexagonal crystal structure [(0001) projection] of Cs0.33WO3
Fig.6 Transmittance spectra of tungsten bronze nanoparticles dispersed by 0.01 wt% in toluene.
1. H. Takeda, H. Kuno and K. Adachi: J. Am. Ceram. Soc., 91, 2897 (2008)
2. H. Takeda and K. Adachi: J. Am. Ceram. Soc., 90, 4059 (2007)
3. Atsushi Tofuku and Kenji Adachi: Powder Technology, Vol.2, No.11, p.37 (2011)
4. T. Chonan, A. Tofuku, K. Fujita and K. Adachi: Proc. the Third Int. Conf. on Processing Materials for Properties (PMP III), TMS, 903-908 (2008)
5. K. Adachi, M. Miratsu and T. Asahi: “Absorption and scattering of near-infra-red light by dispersed lanthanum hexaboride nanoparticles for solar control filters,” J. Mater. Res., 25, pp. 510-521(2010).
6. H. Kuno, H. Takeda, and K. Adachi, “Coating solution for forming a selectively transmitting film, a selectively transmitting film and a selectively transmitting multilayer film”, US Patent No. 6060154, 2002.
7. H. Kuno, H. Takeda, and K. Adachi, “Film for cutting off heat rays and a coating liquid for forming the same”, US Patent No. 6277187, 6221945, 2001.
8. K. Adachi, H. Takeda, and H. Kuno, in Proceedings of the 39th Meeting of the 69th Committee of Japan Society for the Promotion of Science, 2002 (Nagoya, 2002) p. 201.
9. H. Takeda, H. Kuno, and K. Adachi, J. Am. Ceram. Soc. 91, 2897 (2008).
10. K. Bange, Solar Energy Mater. & Solar Cells 58, 1 (1999).
11. C. G. Granqvist, Solar Energy Mater. & Solar Cells 60, 201 (2000)
12. K. Adachi and T. Asahi: J. Mater. Res., 27, 965 (2012).
13. H. Takeda, and K. Adachi, “Infrared shielding material microparticle dispersion infrared shield, process for producing infrared shield material microparticle and infrared shielding material microparticle”, US Patent No. 0178254, 2006.
14. H. Takeda and K. Adachi, “Fine particle dispersion of infrared-shielding material, infrared-shielding body, and production method of fine particles of infrared-shielding material and fine particles of infrared-shielding material”, US Patent No. 8083847, 2011.
Ready to get started? Contact us to talk about your requirements.







![[Wet-chemical synthesized metal powder] Ultra-fine nickel powders](https://crossmining.smm.co.jp/wp-content/uploads/m_np.webp)

