Having extensive experiences in handling a variety of metals in the liquid phase, SMM synthesize inorganic particles by the liquid phase method.
table of contents
Particulate Synthetic Method
There are generally two methods for manufacturing particles: crushing and synthesizing. The synthetic method can be broadly divided into the gas method and the liquid phase method.
Features of the liquid phase method
The liquid phase method is a particle synthesis method that makes it easy to obtain the desired particles. This method is to dissolve the substance that is the source of the particles in a liquid (solution), and then evaporate, cool, or react the solution. These operations are generally known as “crystallization.”
As will be detailed later, this method has a wide range of controllable operations so the shape and size of particles can be changed.
Crystallization of Metals
We are manufacturing nickel(Ni) and copper(Cu) particles using a method called “reaction crystallization”. This is one of the liquid phase methods and fine particles are synthesized by reduction.
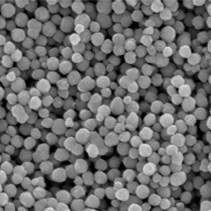
Fig.1 SEM photograph of nickel powder (one of our products)
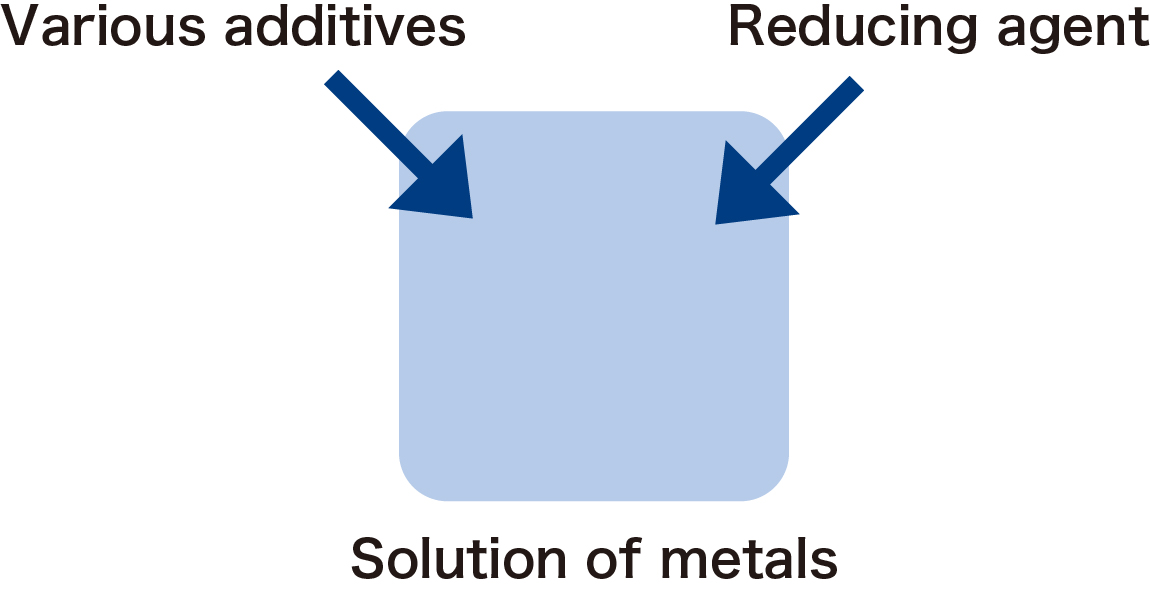
We are manufacturing fine particles of nickel by the reaction of nickel compound and reducing agent. In addition to these main agents, various additives are used to them, and the shape and the size of particles are changed by controlling the reaction conditions (temperature, the timing of putting additives, etc.).
Fig.2 Image of liquid phase reaction
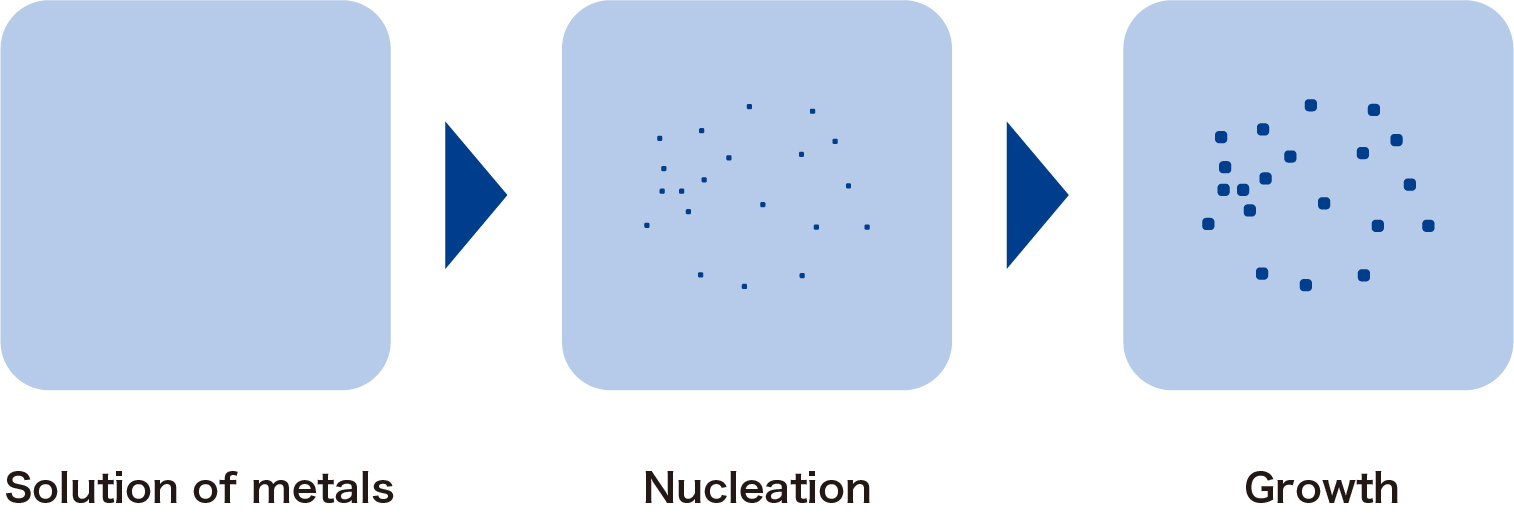
First, the nucleus is generated and grows as a starting point for particle formation. The amount of nucleus generated in this process is the primary determinant of the ultimate size of the particles. How the particles grew up is also the primary determinant of quality, such as the shape and purity of the particles.
Fig.3 Image of reaction process (Nucleation => Growth)
There are several types of nucleation methods, such as adding a pre-made nucleus and generating nucleus in solution at the beginning of the reaction. What is important is generating and to grow the nucleus uniformly. For example, if we successfully create a uniform nucleus and put it in, it will be no meaning if it melts or sticks to each other before it grows. After creating a uniform nucleus the next challenge is how to grow them. Adjusting various conditions is required to generate the desired shape and size of the particles.
It is not a reaction crystallization, but as an example please imagine how salt water is boiled down to make salt crystals. Have you ever done a science experiment to make salt by hanging a small square crystal with thread in a saline solution that is no longer soluble? This small crystal is the nucleus. After that, if the saline solution is slowly evaporated (grown) it will become a large, square, clean salt crystal, but if it is boiled in a hurry, it will become a small bulk of salt. In this case, the failure to control growing conditions (speed of evaporation) could have resulted in the unintended consequence. Because a nucleus different from the original one was generated in a different place.
That’s just an introduction to crystallization, but I hope you understand the importance of controlling various conditions. In other words, it is a feature of the liquid-phase method including crystallization that various particles can be obtained depending on the conditions.
Take part in X-MINING Contact Us
Ready to get started? Contact us to talk about your requirements.
X-MINING provides updates on SMM's material products and information on participating in events. Please register.











![[Wet-chemical synthesized metal powder] Ultra-fine nickel powders](https://crossmining.smm.co.jp/wp-content/uploads/m_np.webp)

